Caractérisation moléculaire et étude clinique des infections au virus Aichi détecté chez les enfants atteints de gastroentérite en Tunisie : combinaison avec l’étude de la séroprévalence (IgG anti -virus Aichi) dans la population. Khira Sdiri-Loulizi1,2*, Mouna Hassine1, Jean-Baptiste Bour2, Katia Ambert-Balay2, Ludwig-Serge Aho3, Nabil Sakly4, Slaheddine Chouchane5, Mohamed Neji-Guédiche5, Pierre Pothier2 and Mahjoub Aouni1 1: Laboratory of Infectious Diseases and Biological Agents, Faculty of Pharmacy, TU-5000 Monastir, Tunisia; 2: National Reference Center for Enteric Viruses, Laboratory of Virology, University Hospital of Dijon, F-21070 Dijon, France 3: Hygiene and Epidemiology, University Hospital of Dijon, BP 77908, 21079 Dijon, France ; 4: Laboratory of Immunology, University Hospital Fattouma Bourguiba, TU-5000 Monastir, Tunisia; 5: Pediatric Department, University Hospital Fattouma Bourguiba, TU-5000 Monastir, Tunisia Objet de l'étude : Les gastroentérites aiguës virales constituent un problème de santé publique mondial avec une morbidité et une mortalité importantes chez le jeune enfant, principalement dans les pays en voie de développement. Les objectifs de cette étude étaient de préciser la fréquence du virus Aichi chez les enfants souffrant de gastroentérite en Tunisie, déterminer l’épidémiologie moléculaire et l’aspect clinique de la diarrhée infantile associée à ce virus émergeant et établir la corrélation entre ces données et l’étude de la séroprévalence du virus Aichi dans la population tunisienne. Méthodes : Une étude prospective a été menée entre janvier 2003 et avril 2007 chez 788 enfants de moins de 12 ans consultant ou hospitalisés au CHU de Monastir pour une gastroentérite aiguë. Le virus Aichi a été identifié et caractérisé par RT-PCR suivi d’étude phylogénétique. Pour l’étude de la séroprévalence, les sérums de 1000 patients (6 mois à 89 ans) ont été collectés et analysés par ELISA pour rechercher les IgG spécifiques anti-virus Aichi. La prévalence (IgG) et l’intensité du signal (titre des sérums) ont été analysées dans les sérums positifs. Résultats : Parmi les 788 selles d’enfants atteints de gastroentérites, le virus Aichi a été détecté dans 32 (4,1 %) échantillons dont 25 cas sont des monoinfections. Les 32 souches du virus Aichi sont de génotype A. Le virus Aichi (P = 0,04) était significativement plus fréquent chez les enfants hospitalisés (4,4%) que chez les externes (1,8%). La comparaison des signes de gravité entre les infections dues au rotavirus et celles dues au virus Aichi ainsi que les autres virus ne montre pas de différence significative (P > 0,05). L’étude de la séroprévalence a montré que les anticorps (IgG) spécifiques anti-virus Aichi augmentent avec l’âge : de 68,8% chez les enfants de moins de 10 ans jusqu’à 100% chez les personnes âgées de plus de 60 ans. Le titre d’anticorps augmente significativement avec l’âge des patients. Conclusion : La corrélation entre la séroprévalence très élevée et la forte fréquence du virus Aichi montre que les infections par ce virus sont importantes et répétées, ce qui reflète l’importance et le caractère émergeant du virus Aichi dans l’étiologie virale des gastroentérites infantiles. Mots clés : gastroentérites virales ; virus Aichi ; caractérisation moléculaire ; séroprévalence ; pédiatrie ; Tunisie Aichi virus IgG seroprevalence in Tunisia: parallel with genomic detection and clinical presentation in children with gastroenteritis Khira Sdiri-Loulizi1,2*, Mouna Hassine1, Jean-Baptiste Bour2, Katia Ambert-Balay2, Ludwig-Serge Aho3, Nabil Sakly4, Slaheddine Chouchane5, Mohamed Neji-Guédiche5, Pierre Pothier2 and Mahjoub Aouni1 1: Laboratory of Infectious Diseases and Biological Agents, Faculty of Pharmacy, TU-5000 Monastir, Tunisia 2: National Reference Center for Enteric Viruses, Laboratory of Virology, University Hospital of Dijon, F-21070 Dijon, France 3: Hygiene and Epidemiology, University Hospital of Dijon, BP 77908, 21079 Dijon, France 4: Laboratory of Immunology, University Hospital Fattouma Bourguiba, TU-5000 Monastir, Tunisia 5: Pediatric Department, University Hospital Fattouma Bourguiba, TU-5000 Monastir, Tunisia E-mail: khiraso@yahoo.fr ABSTRACT Aichi virus has been described as a novel causative agent of gastroenteritis in humans. In this study we report the seroprevalence distribution of Aichi virus in Tunisia. A panel of 1000 sera was screened applying an enzyme-linked immunosorbent assay for immunoglobulin G specific to Aichi virus. A considerable prevalence (92%) of antibody to Aichi virus was found across all age groups. The specific anti-Aichi virus antibodies increased with age, from a high rate (68.8%) in children under 10 years old to about 100% in persons more than 60 years old. We found a statistically significant increase in antibody levels to Aichi virus according to age of patients. Immunoglobulin M antibodies were detected among five children. A high frequency of Aichi virus monoinfections in hospitalized children with severe gastroenteritis was previously observed in Tunisia. Aichi virus cause diarrhea with dehydration, fever and vomiting. This work is the first to establish a correlation between the high seroprevalence of specific Aichi virus antibodies, clinical presentation and high frequency isolation of Aichi virus by genomic characterization in stools of children suffering from gastroenteritis. Our data show the importance and emerging character of the Aichi virus in the viral etiology of the pediatric gastroenteritis. Keywords: Aichi virus; IgG seroprevalence; symptoms; gastroenteritis; Tunisia INTRODUCTION Viral gastroenteritis is a common illness that affects humans worldwide. Rotavirus, calicivirus, adenovirus and astrovirus have been established as the most important etiologic agents in these clinical diseases (5). Nevertheless, for many non bacterial gastroenteritis cases no etiological agent is diagnosed, and it has been hypothesized that other viral agents are involved. Among these, Aichi virus (AiV) was first recognized in 1989 as the likely cause of oyster-associated gastroenteritis in a Japanese patient (16). This virus is a new member of the Picornaviridae family and classified in a new genus named Kobuvirus (10, 16). The detection of AiV in stool samples collected from nonbacterial gastroenteritis outbreaks due to oyster consumption was documented in Asia (15, 17, 20) and in Europe (1, 8). Moreover, this virus was recently identified in oysters implicated in gastroenteritis outbreak in France (7). The detection of AiV strains has also been reported in fecal specimens of children suffering from gastrointestinal symptoms in several studies in Asian countries (9, 18, 21) in Brazil (8) and in France (1). However, a low incidence (0.9 - 3.1%) of AiV strains was observed in all these survey in sporadic as in epidemic gastroenteritis. On the other hand, several seroprevalence studies of antibodies to the AiV has been conducted in Japan (17), Germany (8) and France (6) showing a high level of seroprevalence (80-95%) in adults, which support the widespread exposure to AiV at least during childhood and prove that this virus is quite frequent. In Tunisia, we previously reported the epidemiology and genomic characterization of AiV strains circulating in the pediatric Tunisian population during more than 4 years (13). In this previous prospective survey, contrary to the data of the literature, we showed that AiV was the third most frequent agent detected after rotavirus and norovirus in children with sporadic gastroenteritis symptoms. In addition, we observed the high incidence of monoinfections and the relatively high frequency of hospitalisations due to AiV infections, indicating the role of AiV as a causative agent of pediatric diarrhea in our country. Moreover, we previously analysed sewage and shellfish samples for the presence of AiV from January 2003 to April 2007 (Submitted) and we performed a comparative analysis of environmental AiV strains with those from clinical cases detected at the same period. AiV was the second most frequent pathogen in sewage after rotavirus and a correlation between environmental and human strains was observed. These previous data suggest that AiV play an important role in pediatric gastroenteritis and environmental contamination in Tunisia. Pursuing our research on the AiV epidemiology, in this paper we report the first seroepidemiological survey of antibodies to AiV in Tunisian population. One thousand (1000) randomly chosen sera from Tunisian individuals were analysed by an enzyme-linked immunosorbent assay (ELISA) for immunoglobulin G (IgG) specific to AiV. We performed a statistical analysis of IgG antibody levels according to age and we also looked for AiV-specific immunoglobulin M (IgM) antibodies. We combined the serologic results with clinical and virological data in order to better understand the epidemiology and the role of AiV as pathogenic agent implicated in gastroenteritis disease. MATERIALS AND METHODS Serum samples. A total of 1000 sera were randomly collected from Tunisian subjects who consulted at University Hospital of Monastir, Tunisia, between January and February 2007. Serum samples were selected from patients grouped into the eight following 10 year age ranges: 6 months to 10 years (n =106; 10.6%), 11 to 20 years (n = 156; 15.6%), 21 to 30 years (n = 129; 12.9%); 31 to 40 years (n = 118; 11.8%); 41 to 50 years (n = 115; 11.5%); 51 to 60 years (n = 130; 13%); 61 to 70 years (n = 129; 12.9%) and 71 to 89 years (n = 117; 11.7%). In our study the population was homogeneous. After their arrival in the laboratory, the sera were immediately stored at -20°C. Cell culture and viruses. AiV strain (A846/88) was kindly provided by T. Yamashita. Vero cells were grown in minimal essential medium (MEM, GIBCO) supplemented with 10% fetal calf serum (Eurobio), 1% Eagle’s non-essential amino acids (100X, Eurobio), 1% glutamine (200 mM, Sigma) and 1% antibiotics (penicillin 5000 UI/ml, streptomycin 5000 µg/ml). As previously described (6), AiV was grown on Vero cells (at 37 °C, 5% CO2). Viral antigen was prepared by clarification of cell lysates (2,465 g, 20 min, 4°C), then titrated at 107 TCID 50/mL and stored at -80°C. Serological study. The detection of AiV- specific antibodies was performed by an enzyme-linked immunosorbent assay (ELISA). Mock and infected (Aichi) Vero cells were identically prepared and each sample was also tested on Aichi and mock antigen. A 96-well microtiter plate (cat. no. 469949; Nunc, Maxisorp) was coated with antigen diluted at 1/20 in PBS (pH 7.4) for 1h at 37 °C (100 µl into each well). Then the wells were blocked with 150 µl of 3% dry skim milk in PBS (Instant Skimmed Milk powder cat: 2920990) at 37°C for 30 min. The supernatants (blocking reagents) were rejected and 1/100 dilutions of serum samples were distributed into wells and incubated for 30 min at 37°C. The plate was washed 5 times by PBS/Tween 20 (0.1%). Then 100 µl of the HRP-conjugate Mouse Anti-Human IgG (Cat. No. 9040-05, SouthernBiotech) were dispensed into the wells (at 1/20.000 in PBS) and the plate was incubated at 37°C for 30 min. This was followed by 5 rinses. Enzymatic activity was revealed by the TMB peroxidase substrate (3, 3’, 5, 5’-tetramethylbenzidine (KPL, Eurobio)). The reaction was blocked after 10 min by 50 µl of stopping solution (H2SO4 , 1N, Bio-Rad) and the optical absorbance was read at 450 nm with 620 nm as reference. In order to align the optical density (OD) of samples, we added, in each run, the same negative (human serum with absorbance value below PBS) and positive (human serum sample with high antibody titer) calibrators to mock and Aichi plates. We calculated the mean of absorbance given on Aichi plates by the positive calibrator for each run. We then determined, for each run, the deviation of its calibrator to this mean in order to redress the absorbance values of each serum in each run. The cut-off was determined on the basis of backgrounds obtained on Mock plate. Briefly, OD was redressed as above by mean of negative calibrator and the distribution of corrected OD was analyzed showing that 95% of samples were below OD = 0.125. Thus, we considered as positive a sample with absorbance value greater than 0.125 on viral antigen. Signals obtained by this ELISA has been previously shown to be well correlated to those given by neutralization test (6). Serological detection of a recent episode of AiV infection. The diagnosis of recent AiV infection was based on the detection of specific IgM antibodies. In this work, 72 ELISA-positive sera were randomly selected in the children aged 6 months to 10 years (n = 106) and assayed for IgM antibodies specific to AiV by a direct immunofluorescence assay (IFA) on infected Vero cells. Briefly, serum (1/50 in PBS) was added on slides supporting Aichi-infected Vero cells and incubated 30 min at 37°C. After 4 rinses by PBS, a fluorescein conjugated anti-human IgM (Bio-Rad) was added and incubated 30 min at 37°C. Slides were then rinsed as above and microscopically observed under UV light. Positive sera showed a typical picture of infected cells containing cytoplasmic granular inclusions. In order to avoid false-positive results each positive serum was assessed by the FIDIS Rheuma-RF kit (Biomedical Diagnostics, Marne la Vallee, France) which detects rheumatoid factors (IgM anti-IgG). This assay was performed according to the manufacturer’s instructions. Stool samples and clinical data. From January 2003 to April 2007, a prospective study was previously conducted in Tunisia and concerned stool samples collected from 788 children (413 males and 375 females) under 12 years of age who suffered from acute gastroenteritis. Four hundred eight (408) samples were collected from children within 48 h following their hospitalization for acute gastroenteritis in Monastir University Hospital (inpatients), and 380 samples were collected from children presenting in the dispensaries for gastrointestinal symptoms (outpatients). The samples were screened for routine bacterial agents and parasites and then stored at - 20°C for further analyses. Cases were identified by reviewing hospital admission logs for demographic characteristics of the patients (name, age, sex, etc.) and symptoms. Clinical data involving such disease manifestations as fever, vomiting, abdominal pain, or bloody diarrhea were collected for all patients. Severity criteria, such as duration of the diarrhea, number of stools or bouts of vomiting, range of body temperature, degree of dehydration, capillary refill time (CRT), and the presence of skin blotches, were determined for all hospitalized children as previously documented (12). Statistical analyses. The statistical analyses were performed with STATA Software (version 8). Seroprevalence levels were compared by means of 95% confidence intervals established using the binomial exact method. Distribution of ELISA-positive IgG levels (IgG antibodies anti AiV) for each age group were analyzed by the Kruskall-Wallis test and non-parametric trend test. P values ≤ 0.05 were considered significant. RESULTS Seroprevalence of AiV antibodies The seroprevalence of specific antibodies to AiV was determined in a total of 1000 human serum samples randomly collected from Monastir, Tunisia, using indirect enzyme-linked immunosorbent assay. Our survey shows a high seroprevalence of AiV in Tunisian population aged from 6 months to 89 years. Among the 1000 analyzed sera, 917 (92%) were positive, containing the IgG antibodies to AiV. Based on ELISA results, seroprevalence was calculated for each age group (Fig 1): the prevalence of antibodies to AiV increased with age, rising from 68.8% [6 months-10 years] to about 100% [71-89 years]. Seroprevalence of AiV antibodies progresses statistically with the age; in the age group [21-30 years] the seroprevalence of AiV antibodies was significantly different (P < 0.05.) compared to the preceding age groups [6 months-10 years] and [11-20 years]. Seroprevalence showed no significant variations from [31-40 years] to 89-year-old patients (P < 0.05). Age distribution of AiV-specific IgG antibody levels We studied the distribution of IgG ELISA-positive levels among age groups in Tunisian population (Fig.2). The statistical analyses of our data show that the distribution of signal intensity of AiV-specific IgG antibodies among age groups was not homogeneous. Logistic regression analysis was used to compare the 917 values of OD by using as age group-reference the 24 babies < 1 year of age: [<1 year]. Our data show that IgG antibody levels increased with age: in the young children [1-10 years] and in the 11 to 20-year-old group IgG levels were low and no significant difference was observed in these age groups with our reference group (P = 0.221 and P = 0.794, respectively), whereas, starting from age [21-30 years] group the difference was statistically significant (Kruskall-Wallis test, P = 0.01) compared to the reference group. We also found that the rate of significance tends to increase according to the age (ex: P = 0.002 in [41-50 years] and P = 0.001 in [51-60 years]. Detection of specific IgM antibodies to AiV In this work, among 72 ELISA-positive sera (IgG antibodies to AiV strains) randomly selected in the children aged 6 months to 10 years (n = 106), 5 sera were positive for IgM antibodies by IF assay (5 children from 6 months, 3, 5, 6 and 10-year-old). These 5 sera were tested by the FIDIS Rheuma-RF kit, all of them showing negative results (RF < 25 IU/ml). Virological and clinical data of AiV in Tunisia Our serological data were combined with clinical survey previously documented in our country between January 2003 and April 2007 involving children suffering from gastroenteritis symptoms (13). In this clinical study, stool samples were all negative for bacterial pathogens and parasites (12). Our previous data showed that 32 samples were positive for AiV. Among monoinfections to Aichi virus (n=25), 18 were detected in stool samples from children suffering from severe diarrhea requiring hospitalisation (inpatients) (Table 1). We observed that age of children mono-infected by AiV strain was between 3 month and 9 years. Among these 18 AiV monoinfection cases, 9 children had fever (T ≥ 37.5°C), among them 4 children (between 6 month and 9 year-old) had fever ≥ 39°C, 15 children had CRT (capillary refill time) < 3s and 3 children had CRT ≥ 3s (3; 4 and > 5s). Of the 18 hospitalized pediatric patients, 17 of these essentially recovered (good rehydration, absence of diarrhea and/or vomiting). The last one was a 6-month-old girl who died during the hospitalization. She presented several criteria of severity: diarrhea lasted for 14 days with frequency of 5 stools /24 h, vomiting (4 episodes /24 h), fever (39°C) and she suffered from dehydration (degree 2; 5-10%). Her CRT was > 5 seconds and skin blotches were observed. During her admission on August 13th, 2006, this child suffered from gastroenteritis symptoms, after 3 days of hospitalization she died on August 15th, 2006. Our clinical data showed also that among these 18 children mono-infected by the AiV strain, two children with gastroenteritis symptoms had anemia. To note is the only patient hospitalized for acute gastroenteritis who presented a mixed infection with AiV: The diarrheic stools were also contaminated by rotavirus. This 28 month old child died during his hospitalization in the pediatric service. At the time of admission this patient suffered from diarrhea and vomiting; the diarrhea lasted 5 days with 4 stools per day and 5 vomiting episodes per day. DISCUSSION To our knowledge this is the first study on the African continent and especially in Tunisia to analyse seroprevalence of Aichi virus. Our results show a high prevalence of antibodies against AiV in the Tunisian population (92%), higher than observed in previous studies conducted in France (77%) Germany (76%) and Japan (55%) (6, 8, 17). Of the 1000 individuals pertaining our study, 83 (8.3%) persons remained non reactive for IgG antibodies, suggesting that, at the time of testing, they really have had no contact with AiV. The distribution of the seroprevalence according to the age groups was not homogeneous. This seroprevalence was age-dependent, increasing with age from 68.8% [6 months-10 years] to about 100% [71-89 years]. This progression has been observed in other countries in Europe and in Japan (2, 6, 8, 17). Our data are the first to show such a high seroprevalence (about 69%) in young children (<10 year old). Indeed, previous reports showed a high seroprevalence in teen and young adults, but limited seroprevalence in younger children (7% in Japan and 25 % in France) showing that in these countries AiV is thought to primary infect older persons. Thus gastroenteritis due to AiV was suggested to mainly affect persons aged 15 to 34 years (6, 17). For babies, the maternal antibodies could be a bias in seroprevalence studies (6, 8). However in our work we measured the seroprevalence in the first age group by removing the children less than one year old (n = 24) and we did not find any significant difference between these two sets. This suggests that positive children have been infected by AiV and produce specific antibodies in their sera. The differences of patterns of IgG antibodies distribution in these countries could indicate a variation in AiV epidemiology. In Tunisia, seroprevalence study showed no more progress in antibody rate in adults and no significant variation from [21-30 years] to 89-year-old patients (plateau at around 95%). These data suggest that seroconversions due to primo-infection by AiV occurred in childhood or adolescence and in any case before the age of 30 years. This seroprevalence profile corresponds partly to that observed in several countries: Seroconversions occur before the age of 40 in France (84% of positive sera at 30 years of age) (6) as in Japan (83.3% of positive cases at 35 years of ages) (17); in Germany, primoinfection occur before the age of 20 (86% of positive sera at 15 years of age); in Spain 60% of the population is infected before 20 years of age and 93% before 40 years of age (2). The high level of seroprevalence in adults showed in several reports suggests a widespread exposure of populations to AiV (6, 8, 17). In this work, the analysis of the positive IgG antibody levels by age showed that signal intensity of IgG antibodies in positive sera (n = 917) correlated with age. Indeed, the IgG levels of specific anti-AiV antibodies increase gradually and statistically with age; the youngest patients have the lower IgG antibody levels and starting from age group [21-30 years] the difference was statistically significant (Kruskall-Wallis test, P = 0.01) compared to patients < 1 year of age. These results show that sera were more reactive in persons older than 30 years which indicates an anamnestic humoral response, due to reinfections even asymptomatic. Among 72 sera tested for IgM antibodies, 5 were positive and all of them showed negative results in Rheumatoid factor test (IgM anti-IgG). Confirming that these IgM were specific against AiV. As IgM antibodies are the serological marker of recent primary infection, their presence in sera indicates the possibility of a recent infection for the 5 corresponding children during the survey, even if such antibodies may be present in the serum up to about 18 months after the first infection (3, 4). It is interesting to find sera positive for specific AiV-IgM antibodies. Indeed the detection of these IgM specific to AiV was very rare as T.Yamashita documented some AiV infections without any detection of anti-AiV IgM during nine outbreaks of gastroenteritis (17). As well the French study reported no IgM among positive IgG sera even at 1/10 dilution (6). In our previous clinical and virological study (13), AiV genomic detection was relatively higher than usually documented: it was found in 32 (4.1 % of diarrheic stools ) cases with a high proportion of monoinfection (25; 78.1% of AiV infections) and a high frequency of hospitalisations among the monoinfections (18 versus 7 outpatients ; P = 0.04). The 18 children hospitalized presented gastroenteritis symptoms, especially diarrhea, fever and vomiting. Severity criteria, such as dehydration, CRT, the presence of skin blotches and even a case of death were observed in these children monoinfected by AiV. These data support the role of AiV in pediatric gastroenteritis in our country as a real pathogenic agent virulent enough to require hospitalisation. These previous results are concordant with those from our serological analysis showing a high prevalence of IgG anti AiV antibodies in children ≤ 10 years (68.8%) confirming the frequent contact with AiV infection in this age group. Although the AiV pathogenicity was considered as doubtful, for a long time, and AiV was evaluated as a virus without danger, causing asymptomatic infections or symptoms not severe enough to require medical attention (14, 19, 20). Our data prove that this virus is really implicated in severe diarrhea causing hospitalization of children. Thus the profile of AiV infection seems to be different in Tunisia and serological and clinical presentation could reinforce the ideas concerning the pathogenicity of this emerging virus. In the same way a recent study conducted in Hungary showed that AiV was detected in a child with clinical symptoms as diarrhea, fever, purulent conjunctivitis and respiratory symptoms (11). This work is the first to establish a correlation between the high seroprevalence and the high frequency of AiV isolation and shows that both clinical and virological features of AiV differ between Tunisian and Japanese or European populations, all other studies showing discrepancies between the relatively high seroprevalence (2, 6, 8, 17) and the low AiV detection rate in sporadic cases and outbreaks of acute gastroenteritis (1, 8, 9, 11, 14, 18). Moreover, for Tunisian children mono-infected by Aichi Virus (13) there was no history of any travel abroad or eating of seafood before the onset of their illness. In addition, in Tunisia seafood is not commonly consumed but generally intended for export. Thus, oysters and shellfish can be excluded as principal source of general AiV exposure as previously reported (16). These data suggest that AiV can be transmitted by other ways. We think that, like other enteric viruses, AiV are probably transmitted through the fecal-oral route: directly by person to person contact or indirectly via fomites, contaminated food or water, knowing that AiV strains were detected with high frequency in raw and treated sewage in our country (personal data). In conclusion, according to our previous results and this serological study, we can consider AiV as an emerging pathogen, very present and requiring to be diagnosed and studied. AiV may be an agent of considerable importance not only in Asian and European countries but also in Africa, and further studies are needed to assess the pathogenesis, immunology and etiological role of AiV in human infection. Our work extends the finding on AiV seroprevalence rates in European and Asian countries to Africa continent, Tunisia. The overall results are similar, confirming infection patterns and adding to our knowledge about the epidemiology of an emerging understudied virus. ACKNOWLEDGMENTS This work was supported by the CMCU Project (code 04/S0813) and the National Reference Center (NRC) for Enteric Viruses, CHU de Dijon (Dijon, France). We thank Teruo Yamashita for kindly providing the prototype strain A846/88 of Aichi virus. We thank Philippe Daval for cell culture preparation and Nils-Olivier Olsson and his team (Immunology Department, CHU de Dijon) for the RF test. REFERENCES
- Ambert-Balay, K., M. Lorrot, F. Bon, H. Giraudon, J. Kaplon, M. Wolfer, P. Lebon, D. Gendrel, and P. Pothier. 2008. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J Clin Microbiol 46:1252-8.
- Buesa, J. 2007. Personal communication, 28 Sept 2007.
- Camargo, M. E., and P. G. Leser. 1976. Diagnostic information from serological tests in human toxoplasmosis. II Evolutive study of antibodies and serological patterns in acquired toxoplasmosis, as detected by hemagglutination, complement fixation, IgG and IgM-immunofluorescence tests. Rev Inst Med Trop Sao Paulo 18:227-38.
- Camargo, M. E., P. G. Leser, and W. S. Leser. 1976. Diagnostic information from serological tests in human toxoplasmosis. I. A comparative study of hemagglutination, complement fixation, IgG and IgM-immunofluorescence tests in 3,752 serum samples. Rev Inst Med Trop Sao Paulo 18:215-26.
- Glass, R. I., J. Bresee, B. Jiang, J. Gentsch, T. Ando, R. Fankhauser, J. Noel, U. Parashar, B. Rosen, and S. S. Monroe. 2001. Gastroenteritis viruses: an overview. Novartis Found Symp 238:5-19; discussion 19-25.
- Goyer, M., L. S. Aho, J. B. Bour, K. Ambert-Balay, and P. Pothier. 2008. Seroprevalence distribution of Aichi virus among a French population in 2006-2007. Arch Virol 153:1171-4.
- Le Guyader, F. S., J. C. Le Saux, K. Ambert-Balay, J. Krol, O. Serais, S. Parnaudeau, H. Giraudon, G. Delmas, M. Pommepuy, P. Pothier, and R. L. Atmar. 2008. A French oyster-related gastroenteritis outbreak: Aichi virus, norovirus, astrovirus, enterovirus and rotavirus all involved in clinical cases. J Clin Microbiol.
- Oh, D. Y., P. A. Silva, B. Hauroeder, S. Diedrich, D. D. Cardoso, and E. Schreier. 2006. Molecular characterization of the first Aichi viruses isolated in Europe and in South America. Arch Virol 151:1199-206.
- Pham, N. T., P. Khamrin, T. A. Nguyen, D. S. Kanti, T. G. Phan, S. Okitsu, and H. Ushijima. 2007. Isolation and molecular characterization of Aichi viruses from fecal specimens collected in Japan, Bangladesh, Thailand, and Vietnam. J Clin Microbiol 45:2287-8.
- Pringle, C. R. 1999. Virus taxonomy at the XIth International Congress of Virology, Sydney, Australia, 1999. Arch Virol 144:2065-70.
- Reuter, G., A. Boldizsar, G. Papp, and P. Pankovics. 2009. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch Virol 154:1529-32.
- Sdiri-Loulizi, K., H. Gharbi-Khelifi, A. de Rougemont, S. Chouchane, N. Sakly, K. Ambert-Balay, M. Hassine, M. N. Guediche, M. Aouni, and P. Pothier. 2008. Acute infantile gastroenteritis associated with human enteric viruses in Tunisia. J Clin Microbiol 46:1349-55.
- Sdiri-Loulizi, K., M. Hassine, H. Gharbi-Khelifi, N. Sakly, S. Chouchane, M. N. Guediche, P. Pothier, M. Aouni, and K. Ambert-Balay. 2009. Detection and Genomic Characterization of Aichi Viruses in Stool Samples from Children in Monastir, Tunisia. J Clin Microbiol 47:2275-2278.
- Svraka, S., E. Duizer, H. Vennema, E. de Bruin, B. van der Veer, B. Dorresteijn, and M. Koopmans. 2007. Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. J Clin Microbiol 45:1389-94.
- Yamashita, T., M. Ito, H. Tsuzuki, and K. Sakae. 2001. Identification of Aichi virus infection by measurement of immunoglobulin responses in an enzyme-linked immunosorbent assay. J Clin Microbiol 39:4178-80.
- Yamashita, T., S. Kobayashi, K. Sakae, S. Nakata, S. Chiba, Y. Ishihara, and S. Isomura. 1991. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J Infect Dis 164:954-7.
- Yamashita, T., K. Sakae, Y. Ishihara, S. Isomura, and E. Utagawa. 1993. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J Clin Microbiol 31:2938-43.
- Yamashita, T., K. Sakae, S. Kobayashi, Y. Ishihara, T. Miyake, A. Mubina, and S. Isomura. 1995. Isolation of cytopathic small round virus (Aichi virus) from Pakistani children and Japanese travelers from Southeast Asia. Microbiol Immunol 39:433-5.
- Yamashita, T., K. Sakae, H. Tsuzuki, Y. Suzuki, N. Ishikawa, N. Takeda, T. Miyamura, and S. Yamazaki. 1998. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J Virol 72:8408-12.
- Yamashita, T., M. Sugiyama, H. Tsuzuki, K. Sakae, Y. Suzuki, and Y. Miyazaki. 2000. Application of a reverse transcription-PCR for identification and differentiation of Aichi virus, a new member of the Picornavirus family associated with gastroenteritis in humans. J Clin Microbiol 38:2955-61.
- Yang, S., W. Zhang, Q. Shen, Z. Yang, J. Zhu, L. Cui, and X. Hua. 2009. Aichi virus strains in children with gastroenteritis, China. Emerg Infect Dis 15:1703-5.
FIGURES AND TABLES Fig. 1. Age distribution of AiV antibodies seroprevalence in a panel of 1000 sera randomly selected from patient Tunisian population (95% confidence intervals). 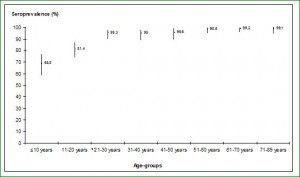 *, starting this age group the difference was statistically significant compared to preceding age groups (P < 0.05). Fig. 2. Sera reactivity against AiV: box plot detailing for each age group the distribution if IgG levels as measured by ELISA
*, starting this age group the difference was statistically significant compared to preceding age groups (P < 0.05). Fig. 2. Sera reactivity against AiV: box plot detailing for each age group the distribution if IgG levels as measured by ELISA 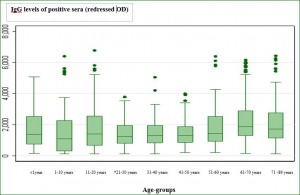 *, starting this age group the difference was statistically significant compared to the reference age group (Kruskall-Wallis test, P = 0.01) Table 1. Clinical and epidemiological study among18 children hospitalized for gastroenteritis and mono-infected by AiV strains.
*, starting this age group the difference was statistically significant compared to the reference age group (Kruskall-Wallis test, P = 0.01) Table 1. Clinical and epidemiological study among18 children hospitalized for gastroenteritis and mono-infected by AiV strains.
| Epidemiological and clinical data of children suffering from gastroenteritis symptoms and infected by AiV | |||||||||||||
| Patient N° | Collect date | Age/ month | Sex | Diarrhea duration (d) | Stool Nb / d | Vomiting Nb / d | Fever* (T°C) | Abdominal Pain | Anemea | Dehydration (degree) | CRT (second) | Skin blotches | State (exit of the hospital)** |
| 333 | 14/02/2003 | 10 | M | 7 | 5 | 3 | 38,5 | 0 | 0 | 1 | < 3" | 0 | 1 |
| 4549 | 21/10/2003 | 15 | M | 5 | 4 | 0 | 36 | 0 | 0 | 0 | < 3" | 0 | 1 |
| 26512 | 22/11/2003 | 29 | F | 4 | 5 | 4 | 39,7 | 0 | 0 | 0 | < 3" | 0 | 1 |
| 27467 | 05/12/2003 | 3 | M | 6 | 4 | 1 | 36,8 | 0 | 0 | 2 | < 3" | 0 | 1 |
| 27581 | 08/12/2003 | 17 | F | 2 | 2 | 3 | 37,2 | 1 | 0 | 0 | < 3" | 0 | 1 |
| 1633 | 24/01/2005 | 9 | F | 2 | 3 | 3 | 37,4 | 0 | 0 | 0 | <3" | 0 | 1 |
| 2403 | 31/01/2005 | 13 | M | 6 | 4 | 3 | 40 | 0 | 0 | 3 | 4" | 1 | 1 |
| 7599 | 02/04/2005 | 8,5 | M | 8 | 3 | 4 | 37,6 | 0 | 0 | 2 | <3" | 0 | 1 |
| 8407 | 12/04/2005 | 11 | M | 6 | 5 | 3 | 36,8 | 0 | 0 | 1 | <3" | 0 | 1 |
| 24459 | 15/10/2005 | 4 | F | 4 | 6 | 0 | 37,5 | 0 | 0 | 2 | 3" | 0 | 1 |
| 24953 | 21/10/2005 | 11 | F | 6 | 7 | 3 | 37,5 | 0 | 1 | 2 | <3" | 0 | 1 |
| 25172 | 24/10/2005 | 24 | M | 2 | 3 | 3 | 37 | 0 | 0 | 1 | <3" | 0 | 1 |
| 26035 | 05/11/2005 | 108 | F | 8 | 9 | 4 | 39,2 | 0 | 0 | 0 | <3" | 0 | 1 |
| 27253 | 18/11/2005 | 4 | M | 6 | 10 | 3 | 37 | 0 | 0 | 2 | <3" | 0 | 1 |
| 28669 | 06/12/2005 | 7 | M | 9 | 5 | 3 | 38,5 | 0 | 0 | 2 | <3" | 0 | 1 |
| 30004 | 20/12/2005 | 13 | M | 4 | 8 | 5 | 37,4 | 0 | 0 | 1 | <3" | 0 | 1 |
| 9406 | 19/04/2006 | 16 | F | 9 | 4 | 4 | 37 | 0 | 1 | 0 | <3" | 0 | 1 |
| 20358 | 13/08/2006 | 6 | F | 14 | 5 | 4 | 39 | 0 | 0 | 2 | >5" | 1 | death |
M: male F: female d: day Nb: number *, Fever: when T ≥37.5°C **, Good health conditions (good rehydration, absence of diarrhea and/or vomiting) = 1




